Testimony Before The Subcommittee On Health Of The House Committee On Ways And Means
Statement of Kris Robinson, Executive Director and CEO, American Association of Kidney Patients, Tampa, Florida
Testimony Before the Subcommittee on Health
of the House Committee on Ways and Means
June 26, 2007
Mr. Chairman, Ranking Member Camp, and members of the Committee, thank you for inviting me before you today to testify. My name is Kris Robinson and I am the Executive Director and CEO of the American Association of Kidney Patients (AAKP) headquartered in Tampa, Florida.
AAKP is the only national non-profit organization founded by kidney patients, for kidney patients. AAKP serves over one million Americans annually who have either lost kidney function (and live with dialysis or transplant) or have chronic kidney disease (CKD). Our organization is dedicated to serving the needs, interests, and welfare of all kidney patients and their families.
And this is the very reason I am here before you today. It was 36 years ago, in 1971, when our organization’s Vice-President, Shep Glazer, made history here in the House Ways and Means Committee Room testifying while he was actually hooked up to a kidney dialysis machine and receiving dialysis. Within a year our government took action, passing landmark legislation in 1972 to cover the costs of kidney dialysis through Medicare.
Mr. Chairman, we thank you for holding this important hearing because, as you know, the government’s policies towards kidney care today have room for improvement. As a kidney transplant recipient myself, I am well aware of the human and financial cost of kidney care. Our nation has the unique opportunity to provide better outcomes for kidney patients – and this can lead to substantial cost savings because better outcomes translate into less reliance on the drugs, dialysis, and hospitalization currently covered by Medicare.
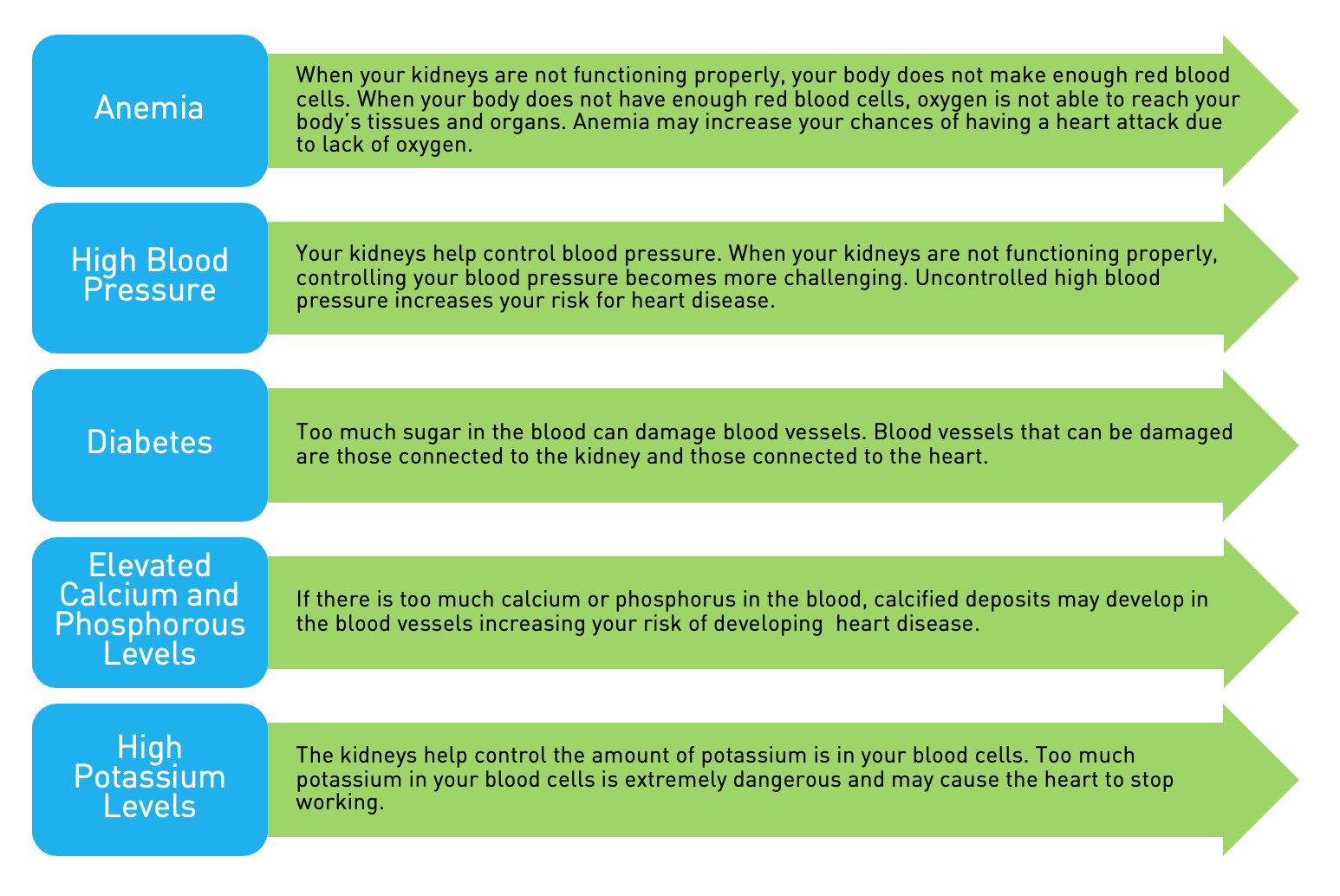
I want to begin by addressing issues regarding anemia management for kidney patients and then also raise several quality improvement recommendations for your Subcommittee’s consideration.
Appropriate Use of ESAs
Let me first stress how important it is to get the dosing of ESAs (erythropoiesis stimulating agents) right for kidney patients. AAKP supports achieving a hemoglobin level of 11 to 12 grams per deciliter, as indicated by the FDA label for ESAs.
We view current CMS monitoring policy as somewhat out of sync with where the FDA is and where the mainstream medical community is. Although each case is different and there will always be outliers, from a patient perspective there is very little medical reason for a patient to remain at levels above 13 grams, especially in light of the current literature citing safety issues. I myself receive epogen for anemia and my doctor would not delay before titrating me down from a level of 13; nor would AAKP’s Medical Advisory Board recommend waiting before doing so.
Yes, we realize that CMS’ monitoring policy is a payment policy and not a policy to set therapeutic targets, but payment policies can often affect decisions regarding treatment options. Since we know overdosing can lead to potentially severe outcomes, we are concerned the current payment policy could provide incentives for overdosing.
Bundling:
Because every medical case is unique, AAKP strongly adheres to the principle that a physician and patient must be permitted to decide a care plan best suited for that patient. Averages and other statistics are fine for certain purposes, but let’s remember that medicine is fundamentally about the treatment of a unique individual.
In this light, we worry about any policy that clouds the doctor/patient decision-making relationship for treatment options. Separate Medicare reimbursement for ESAs potentially distracts from the doctor and patient deciding which course to pursue. That is why we support bundling Medicare reimbursement for ESAs into the overall Medicare composite reimbursement rate for ESRD. We believe that bundling the payment would not only result in cost savings, but also would result in more appropriate dosing of ESAs and draw more attention to the necessarily comprehensive nature of kidney care. It is important, however, to ensure that any bundling structure include risk-adjustment so as not to inadvertently create a disincentive for providers to cover the sickest patients.
ESA Guidelines:
Having said that, let me emphasize that underdosing of ESAs is a danger too. Many kidney patients remember the difficult times before ESAs were available, suffering the debilitating fatigue and adverse health affects associated with anemia. None of us want to return to those days and we do not want to scare patients away from being treated with these valuable life-enhancing medicines. We also do not want to create a perverse disincentive that causes providers to “skimp on” doses of ESAs because they would no longer be receiving separate reimbursement.
What we need is a Medicare policy that strives for a “Goldilocks” solution on ESAs: not too much, not too little, but “just right.”
We believe, therefore, it would be useful to: 1) establish guidelines regarding the proper dosage of ESAs, and 2) link reimbursement to meeting those guidelines. AAKP has long supported linking quality of services to payment for those services.
Subcutaneous Administration of ESAs:
Before leaving the discussion of ESAs, let me say a few words about potential subcutaneous administration of ESAs. As you know, one-third less dosage can be used in subcutaneous administration versus intravenous administration, resulting in substantial cost savings and better outcomes. The Veterans Administration typically administers ESAs subcutaneously.
AAKP surveyed 3,600 patients when the NKF-DOQI guidelines were first released. At that time, DOQI stated that patients should receive their EPO subcutaneously as opposed to intravenously. We surveyed patients concerning the factors they felt doctors should consider when deciding which route (subcutaneous or IV) to administer EPO.
- 93% felt it was “very” or “extremely” important that the doctor make the decision based on “how EPO works best for me.”
- 67% felt that it was “very” or “extremely” important for doctors to consider the patient’s preference with regard to route of administration.
- 74% wanted to be involved in the decision making process.
- Patients also were willing to have EPO administered subcutaneously if they felt it worked best, was more economical, and they could be trained.
- Patients overwhelmingly told us they didn’t mind getting a shot – even giving themselves a shot – if it would make them feel better. Most of these patients are already self-administrating medication due to their diabetes, so one more shot doesn’t faze them.
My point is that our survey of 3,600 patients shows that they would readily accept subcutaneous administration of ESAs. As far as I know, ours is the only such survey data on this question.
Quality Improvement Recommendations
Mr. Chairman, as you know, AAKP has been intimately involved with how kidney care is delivered since the advent of kidney dialysis a generation ago. Based on our 36 years of experience, we offer the following programmatic recommendations for your Subcommittee’s consideration:
- Patient Education:
AAKP is one of the nation’s leading providers of patient education materials and services. Medicare currently does not cover patient education services. We strongly support legislation that would extend Medicare coverage to patient education services and would allow patient education for pre-dialysis patients. The earlier we can start educating patients regarding behavior, nutrition, and other matters in their stages of chronic kidney disease, the fewer health problems will result later. - Standards for Dialysis Technicians:
The quality of services varies considerably in dialysis centers across the country. There is currently no standard for training and certification of technicians in the centers. Some states, like Texas, have strong standards that must be met. Other states, like my state of Florida, have none at all. AAKP would like to see standard training requirements that at least set a minimum for what training dialysis technicians should receive. - Coverage for Home Dialysis:
Dialysis patients typically receive treatment three times a week for four hours a day at a dialysis center. Some patients, however, choose the option of daily home dialysis, which can be administered six times a week for two hours a day. Unfortunately, Medicare only covers three dialysis sessions per week even though more frequent home dialysis can promote better outcomes and save money.Studies show that daily dialysis translates into lower cardiovascular event rates, which is the leading cause of death in kidney patients. Patients undergoing daily dialysis felt much better, especially noting increased energy, better physical functioning, clearer thinking, better control of their anemia and reduced symptoms related to their kidney disease and the dialysis treatments.Daily dialysis can result in savings because: 1) four times as many nurses are needed for conventional dialysis as opposed to home dialysis; 2) hospitalization for daily dialysis patients is reduced by 34%; 3) weekly EPO dosage is reduced by an estimated 41%; and 4) the number of antihypertensive drugs is reduced by 46%. Further, patients undergoing home dialysis have a much greater flexibility in their schedule and are more likely to stay in the workplace. - Lifetime Coverage for Immunosuppressive Drugs:
Medicare coverage for immunosuppressive drugs can expire after 36 months even though kidney transplant recipients need to take the drugs for the rest of their transplanted lives. Many patients who no longer can afford the costs will stop taking the drugs. This leads to graft failures, which cause patients to go back on dialysis and wait for another transplant.Considering that immunosuppressive drug coverage costs approximately $1,000 per month while dialysis costs $4,000 per month and a transplant costs $100,000, it makes fiscal sense to extend Medicare immunosuppressive drug coverage for life. - Extending Medicare Coverage to Stage 4 of ESRD:
Medicare only covers the fifth (and final) stage of ESRD, but this is clearly not in the best interests of the patients. The Renal Physicians Association has stated, “Proactive preparation for RRT (Renal Replacement Therapy) is recommended to facilitate the transition and reduce the burden of clinical risk factors known to be associated with worse outcomes in ESRD patients.” Out of the 28 guidelines the RPA recommends in their physician practice guideline manual, 27 include treatment in both stage 4 and 5, not just in stage 5.A demonstration project would serve to quantify the health and fiscal benefits of stage 4 coverage. - Medicare Coverage for Fistulae Before Stage 5 Eligibility:
The benefits of AV fistular access are already recognized by CMS, who recently enacted a “Fistula First” policy geared towards increasing the number of people who choose this treatment. AAKP strongly endorses the “Fistula First” policy. Fistulae last longer, need less rework, and are associated with lower rates of infections, hospitalization, and death for Medicare beneficiaries than other types of access.However, Medicare coverage does not begin until a patient is at stage 5 of ESRD and an AV fistula should be put in months earlier. We believe this is why fistular access rates are lower than they should be—substantially lower in the United States than in Europe and Japan. Medicare should cover surgical placement of fistulae in stage 4. - Medicare Secondary Payer:
Lastly, AAKP opposes proposals to make Medicare the secondary payer for ESRD services. We believe that the health of patients is enhanced by receiving the comprehensive spectrum of services covered by Medicare. Some proposals would delay Medicare coverage for as long as 60 months. Mr. Chairman, 60 months is five years, and kidney patients in Stage 5 have an annual mortality rate of 25% and a life expectancy of only five years. So making Medicare the secondary payer would mean only the healthiest patients even make it to Medicare coverage. Delaying Medicare coverage increases cost-sharing for patients, and we believe it would undermine patient well-being in many cases.
Mr. Chairman, we applaud your leadership over the years on these issues so important to kidney patients. Our government can vastly improve the quality of care for kidney patients while saving money in many areas. Thank you for having me here to testify today and we offer ourselves as a resource to you for further information as your Subcommittee works on these issues in the months ahead.
Cardiovascular & Renal Drugs Advisory Committee Testimony
Prepared statement from the American Association of Kidney Patients (AAKP) to the FDA’s Cardiovascular and Renal Drugs Advisory Committee given by AAKP’s Vice President Paul T. Conway.
October 18, 2010
My name is Paul Conway and I appear before you today as a patient who has managed kidney disease failure for over 30 years, as a taxpayer and as the Vice President of the American Association of Kidney Patients. I appreciate the opportunity and privilege to participate in this stage of the public policy process and to provide comments on Erythropoiesis-Stimulating Agents or ESAs.
As a kidney patient, my personal journey has been marked by end stage renal disease, long periods of anemia, ESA therapy, nearly two years of peritoneal dialysis, organ transplantation, ongoing immuno-suppression and disease management. Along the way, I have dealt with a multitude of other conditions that stem from this disease. At each stage in the journey, my life has been preserved and extended by skilled teams of specialists, nephrologists, surgeons and nurses who utilized their expertise and knowledge of my health conditions to custom-tailor life sustaining treatments and medication regimens. I am deeply grateful to these selfless heroes. I am also respectful of the untold number of other professionals whose pioneering research, breakthrough medical procedures and pharmaceuticals aided their efforts.
Based on my direct experience, I know the impact of anemia on mental and physical stamina, and the beneficial impact of ESA’s. My views are supported by the experiences of countless other patients from all walks of life whose paths have intersected with mine and through conversations with medical experts across the county.
I respectfully offer three key points to your deliberations as an FDA Advisory Committee. First, we must avoid blood transfusions. Second, dialysis and chronic kidney disease (CKD) patients are different. Third, the decision on what therapy to use must remain between the patient and their doctor, and not usurped by disinterested parties who know nothing about the person who suffers or their makeup as an individual.
Blood Transfusions
Stated simply, ESA therapy has been a breakthrough in dialysis patient management. It has reduced the need for blood transfusions significantly. Transfusions lead to transplantation antibodies, hepatitis, increase hospitalizations, patient and health care costs and loss of personal productivity. A patient with radiation colitis and renal failure would have been hospitalized every week in 1978 for a transfusion. Now, that patient can simply receive ESA injections and maintain a target hemoglobin value without a blood transfusion. The number of transfusions dropped from nearly five hundred per thousand patient years to a little over two hundred between 1992 and 2005 – a change due directly to ESAs.
Dialysis VS Non-Dialysis
Through the years, ESA therapy has evolved as a result of randomized controlled trials, but mainly in the non-dialysis population. It is critically important to match the scientific evidence to the population sampled. When a person is perfectly healthy, he or she may lose around 0.83 cc of blood per day. A CKD patient may lose around 3.15 cc of blood per day and a hemodialysis patient may lose 6.27 cc of blood per day. Thus, patients on dialysis are constantly losing blood, are even more prone to anemia and have a definite need for ESAs.
Individualized Care
Future policies or potential policy modifications regarding anemia should assure that drugs are used safely and that ESA therapy be individualized to the needs of the patient by their medical team. Anemia causes tremendous fatigue in patients with kidney disease.
In my case, before my nephrologist and I agreed to start ESA therapy, it was a challenge to simply get out of bed in the morning, and remaining fully engaged an entire day required tremendous personal discipline. At the time, I served as the Deputy Secretary of Health and Human Services for the Commonwealth of Virginia and both my work and schedule were demanding. Had it not been for ESA therapy and the unwavering support of Governor George Allen and his team, it would have been extremely difficult to continue my duties. ESA therapy allowed me to pursue my commitment to public service and afforded me the ability to contribute to society as a taxpayer instead of tapping assistance that would have rightfully gone to those in greater medical need. Take it from me – the fog and exhaustion caused by anemia can, to paraphrase President Theodore Roosevelt, remove a person from the arena and change them from doers to mere observers.
Studies have shown that raising the hemoglobin from 10 to 12 g/dL increased the Kidney Disease Quality of Life domains for physical activity and for feeling good. It was also shown that the patients in the TREAT trial that had the higher mortality responded poorly to darbepoetin and had the highest incidence of heart disease, implantable defibrillators, strokes and heart attacks.
Thus, patients who are active and healthy with minimal kidney disease may wish to have their hemoglobin around 12 g/dL. Those patients who are sicker, and are resistant to ESAs should have lower hemoglobin. Once again, this decision should be made by the doctor and the patient.
I would like to thank the Advisory Committee for the opportunity to appear here today. On behalf of patients like me and the American Association of Kidney Patients, I would respectfully request that you carefully weigh comments offered by the patients whose lives, families and livelihoods have been positively impacted through the effective use of ESAs.